Acid rain became an “issue” in 1980 when Congress passed the Acid Deposition Act. After a ten year study, National Acidic Precipitation Assessment Program reported that there wasn’t much of a problem with acid rain. The first report was rejected and Congress went ahead and amended the Clean Air Act to mandate SO2 and NOx emissions. These emissions have been substantially reduced at a cost of several billion dollars per year. Chump change relative to the potential costs of Kyoto/Copenhagen schemes.
According to the EPA the Acid Rain Program has made significant progress…
Air Quality: Emission reductions achieved under the ARP have led to improvements in air quality with significant benefits to human health.
- Between 1989-1991 and 2006-2008 average ambient sulfate concentrations have decreased by 38 percent in the Mid-Atlantic, 44 percent in the Midwest, 43 percent in the Northeast, and 28 percent in the Southeast.
Figure 1: Trends in Electricity Generation, Fossil Energy Use, Prices, and Emissions from the Electric Power Industry, 1990-2008
But… The reduction of SO2 and NOx emissions has had no clear affect on the pH of rainwater. Here’s a link to an interactive map of the National Atmospheric Deposition Program…
Click on a station, select “Trend Plots”, then select “Field pH” or “Lab pH” and look at the actual data.
In some parts of the country, the pH is stable, in some parts it’s gently falling, in other parts it’s gently rising. Most stations exhibit little or no change in slope over the measurement period, which in many cases goes back to the late 1970′s.
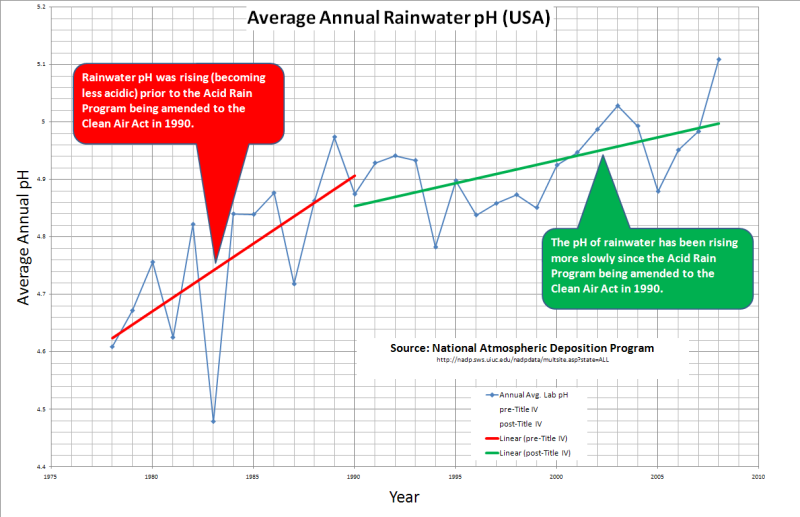
I downloaded all of the annual data from the National Atmospheric Deposition Program and calculated an annual average rainwater pH for the USA…
Rainwater was not becoming more acidic prior to the initiation of the EPA’s Acid Rain Program in 1990. The pH of rainwater was actually rising (becoming less acidic) prior to the EPA’s efforts to fight acid rain. The really crazy thing is that the pH has been rising more slowly since the EPA started to fight acid rain!
Rain is supposed to be acidic. The pH of rain in a pristine environment, free from pollution (including volcanoes) is normally about 5.6. Most of the lakes which were showcased as acid rain victims were naturally acidic and had been acidic since well before mankind ever burned his first lump of coal.
Rather than being a global problem, anthropogenic acid rain was a localized problem in parts of Northern Europe which was relatively easily fixed.
The costs of reducing SO2 and NOx emissions haven’t been that awful… And the reductions did lead to some beneficial incremental environmental effects… But… No acid rain crisis ever existed.
Addendum
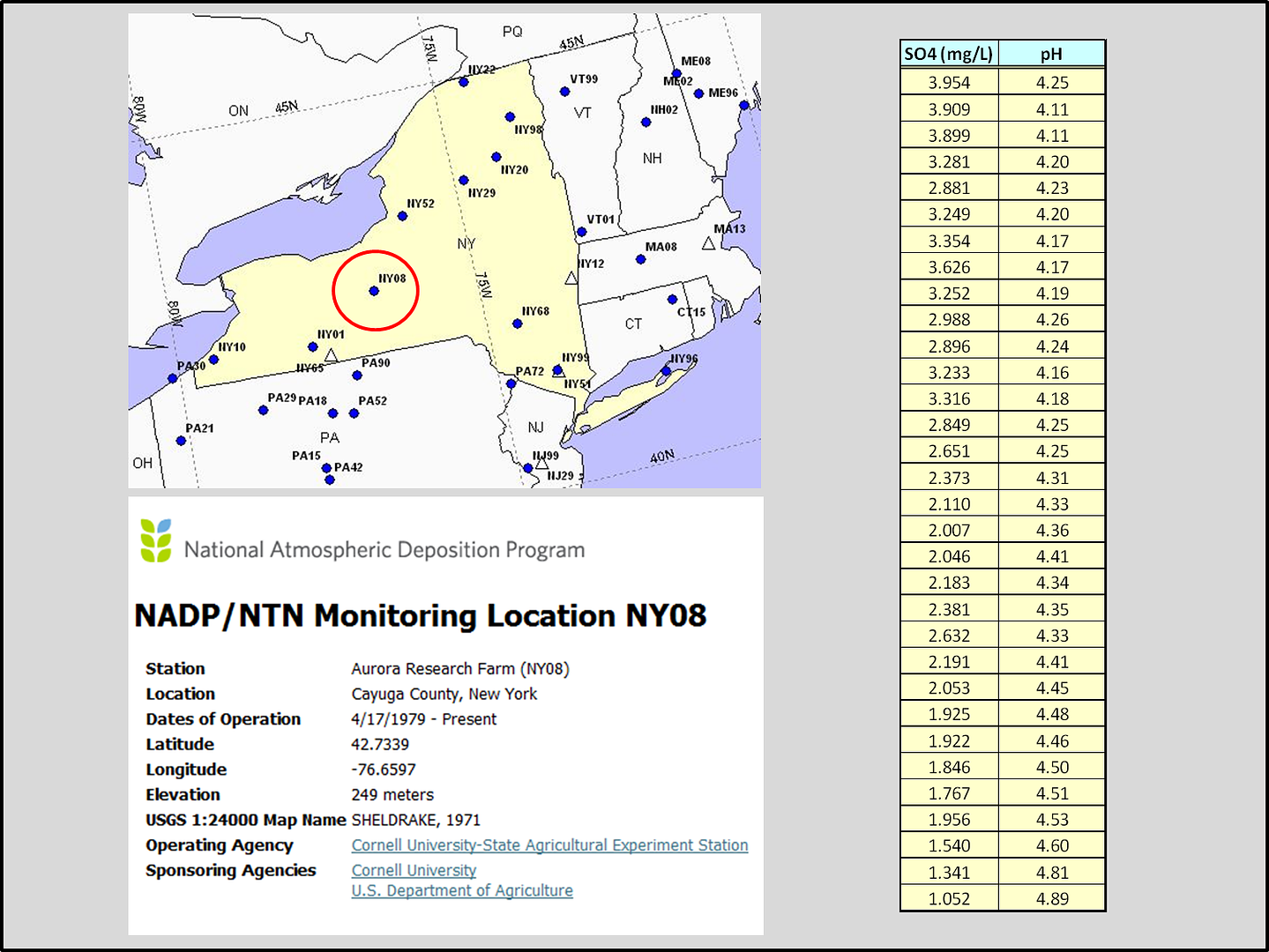
In response to Professor Gutow’s comment: I downloaded a single station from upstate NY with data back to 1979, NY08 Aurora Research Farm…
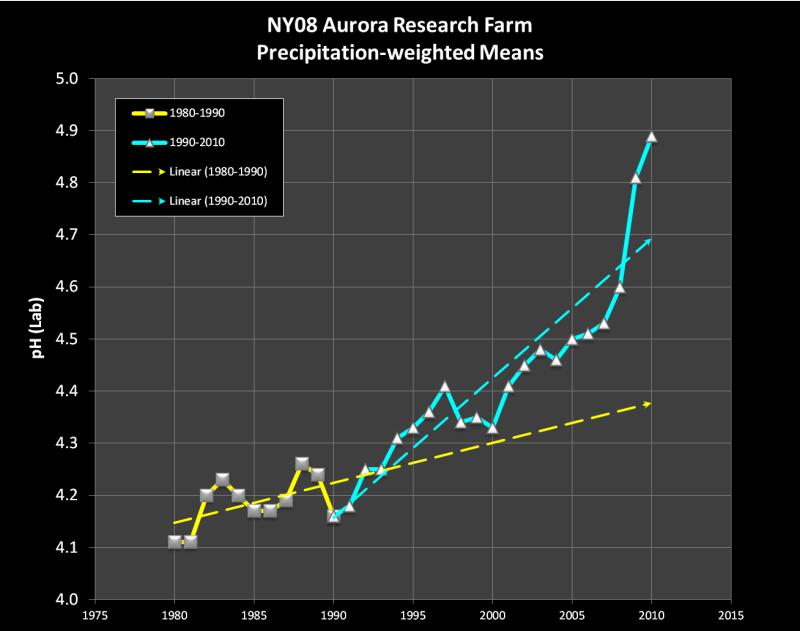
The pH was already increasing before the EPA initiated its Acid Rain Program (ARP)…
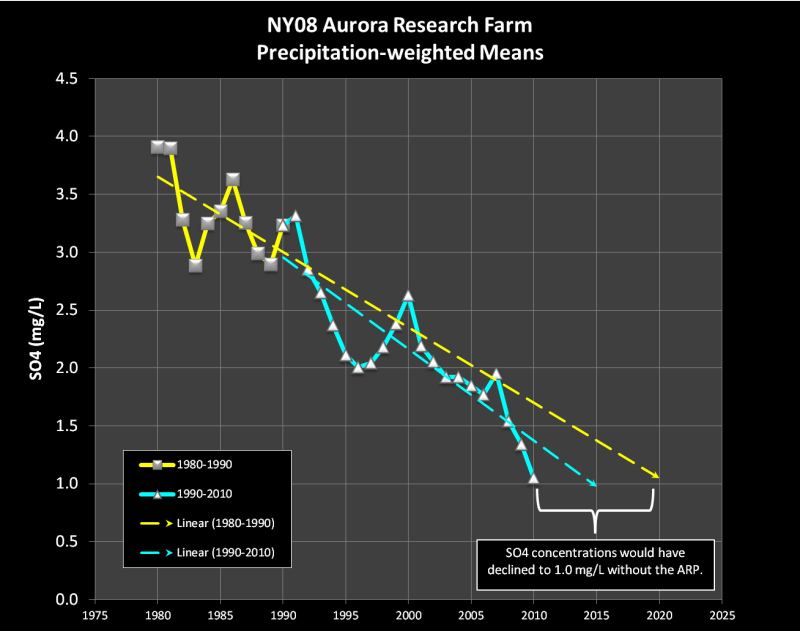
Even more significantly, the SO4 concentration was already in decline. Based on the pre-1990 trend, SO4 would have declined to its current level without ARP, albeit 5 years later…
Propagande des écologistes qui gouvernent l'Europe